![TBST [10X]; Tris buffered saline with Tween-20 (100mM Tris.HCl, 1.5M NaCl, 0.5% Tween-20, pH 7.5) - Cepham Life Sciences Research Products TBST [10X]; Tris buffered saline with Tween-20 (100mM Tris.HCl, 1.5M NaCl, 0.5% Tween-20, pH 7.5) - Cepham Life Sciences Research Products](https://www.cephamls.com/wp-content/uploads/2019/02/TBST-Buffer-10X-1L.jpg)
TBST [10X]; Tris buffered saline with Tween-20 (100mM Tris.HCl, 1.5M NaCl, 0.5% Tween-20, pH 7.5) - Cepham Life Sciences Research Products
TRIS, 500 g, CAS No. 77-86-1 | null | Buffer Solutions and -Salts | Reagents for Histology | Histology/Microscopy | Life Science | Carl Roth - International
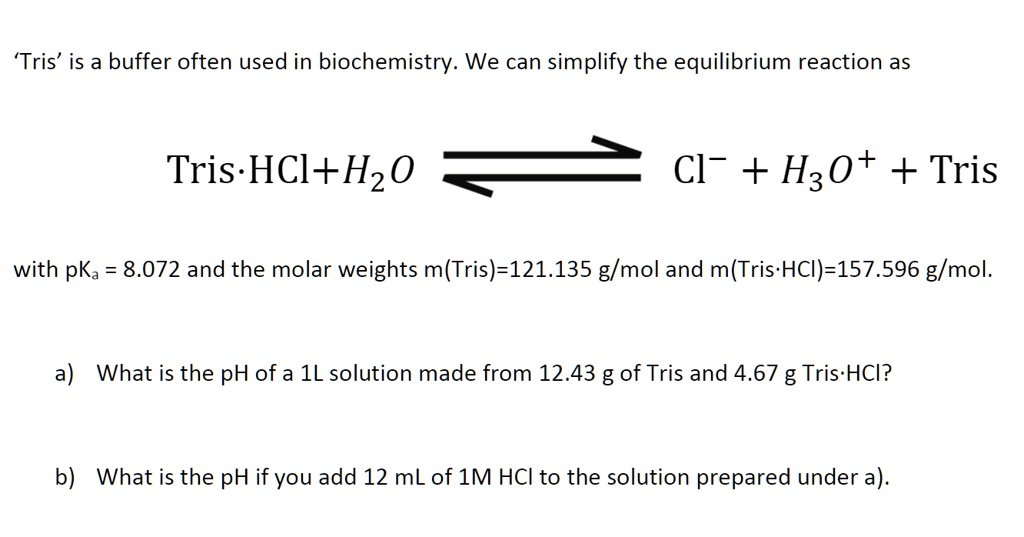
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar
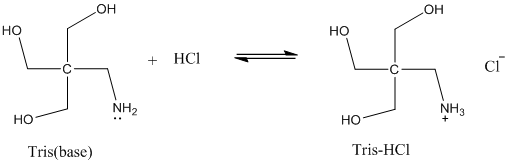
Solved: Chapter 17 Problem 92AP Solution | Loose Leaf Version For Chemistry: Atoms First 2nd Edition | Chegg.com




![T60040-1000.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 1 Kilogram T60040-1000.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 1 Kilogram](https://d2gdaxkudte5p.cloudfront.net/system/images/T60040-1000.0_.jpg)






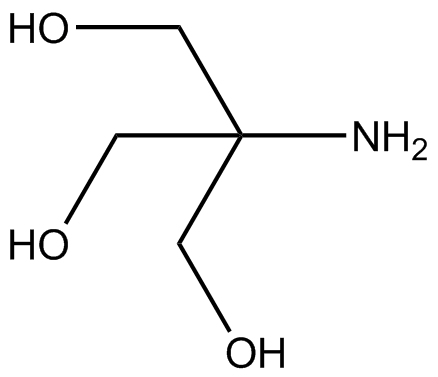
![T60050-100.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 100 Grams T60050-100.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 100 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60050-100.0_.jpg)
![T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60050-500.0.jpg)




