Dissociation of chloramine-T and NaOCl. (A) In the presence of water... | Download Scientific Diagram
Sodium hypochlorite, NaOCl molecule. It contains a sodium cation and a hypochlorite anion. It is used as a liquid bleach and disinfectant. Structural Stock Vector Image & Art - Alamy
SciELO - Brazil - Mechanism of action of sodium hypochlorite Mechanism of action of sodium hypochlorite

Aqueous solution of sodium hypochlorite (NaOCl) is a household bleach and a strong oxidizing agen... - YouTube

Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis | Organic Process Research & Development

SOLVED: Match the following salts with the acid-base neutralization reactants that would produce them: A. NaClO4 = HCl + NaOH B. NaOCl = HCl + NaOH C. Ca(OCl)2 = HCl + Ca(OH)2

Dissociation of chloramine-T and NaOCl. (A) In the presence of water... | Download Scientific Diagram



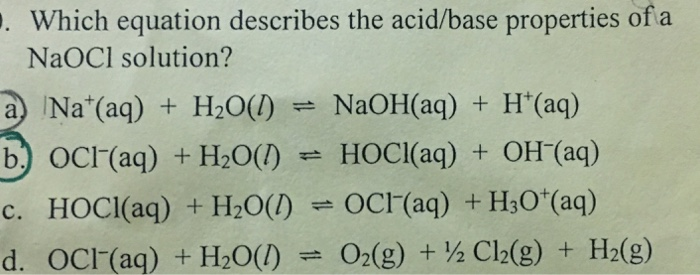

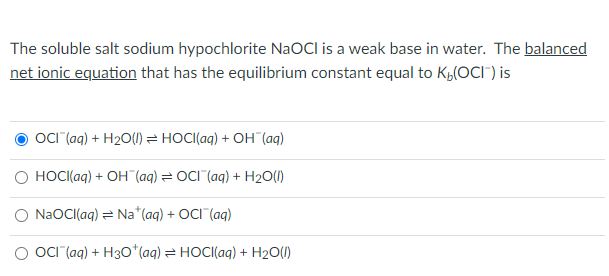

![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)